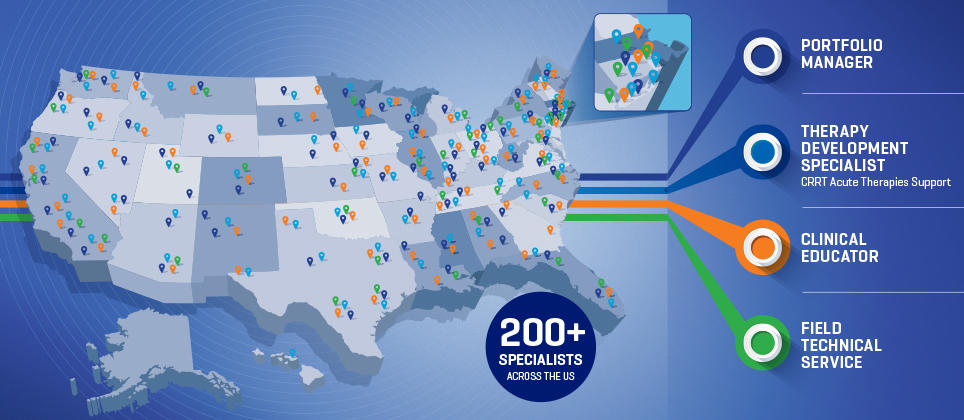
Complete Support
Our dedicated and experienced field team is here to support your program from implementation, ongoing education and training through program assessment and review.
Comprehensive Therapy Implementation
Clinical and Technical Support

24/7 ICON (Intensive Care On-line Network) Clinical Support Help-line
- Provides on-demand 24/7 clinical-focused support service via phone or email
- Staffed by experienced clinical professionals, as well as on-call MDs and PharmDs
- Simulates the situation in question and walks clinician through the process step-by-step
- Supports 1,200 calls monthly from clinicians who are delivering CRRT
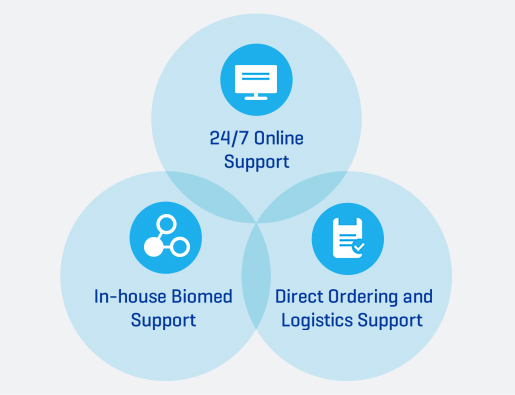
24/7 Technical Support
In addition to your CRRT dedicated team of specialists, we also provide multiple support options. Get answers to your questions any time day or night.
- 24/7 Online Support
- In-house Biomed Support
- Direct Ordering and Logistics Support

Peer-to-peer Training and Education
At Baxter Critical Care Institute, we strive to increase confidence in therapy delivery throughout your hospital. By taking a versatile multi-channel approach to education, our programs are developed to help simplify processes while increasing consistency and safety. We customize training programs to align with the unique needs of your hospital, ICU, and staff. Global insights and knowledge-sharing ensure that our educational offerings are always state-of-the-art.
Indications and Important Risk Information
|
The PRISMAFLEX and PRISMAX Systems are intended for: PHOXILLUM and PRISMASOL Renal Replacement Solution Indications and Important Risk Information Indications PRISMASOL and PHOXILLUM solutions are indicated in pediatric and adult patients for use as a replacement solution in Continuous Renal Replacement Therapy (CRRT) to replace plasma volume removed by ultrafiltration and to correct electrolyte and acid-base imbalances. They may also be used in case of drug poisoning when CRRT is used to remove dialyzable substances Important Risk Information PHOXILLUM and PRISMASOL replacement solutions are contraindicated in patients with known hypersensitivities to these products. PHOXILLUM and PRISMASOL solutions can affect electrolytes and volume and may result in hyperkalemia or hyperphosphatemia. Monitor hemodynamic status and fluid inputs and outputs, potassium, phosphorous, calcium, other electrolytes and acid-base balance throughout the procedure. PHOXILLUM replacement solutions contain hydrogen phosphate, a weak acid that may increase the risk of metabolic acidosis. PRISMASOL and PHOXILLUM replacement solutions can affect blood glucose levels resulting in hypo- or hyper-glycemia depending upon the dextrose content of the replacement solution. Monitor blood glucose levels regularly. The following adverse reactions have been identified during post-approval use with these or other similar products and therefore may occur with use of PHOXILLUM or PRISMASOL: Metabolic acidosis, hypotension, acid-based disorders, electrolyte imbalances including calcium ionized increased, hyperphosphatemia, hypophosphatemia, fluid imbalance. For more information, please see PHOXILLUM and PRISMASOL Solutions full Prescribing Information or visit baxterpi.com. |
|
PRISMASATE Intended Use PRISMASATE is a sterile dialysis solution intended for treatment of acute kidney disease (renal failure) using Continuous Renal Replacement Therapies, such as continuous hemodialysis and hemodiafiltation aimed at normalizing the composition of the blood. Please see PRISMASATE Instructions For Use. MARS Indications MARS is indicated for the treatment of drug overdose and poisonings. The only requirement is that the drug or chemical be dialyzable (in unbound form) and bound by charcoal and/or ion exchange resins. |